β-carotene absorbs light at 453 nm and 483 nm. Calculate the energy, in kcal/mol , corresponding to each of these wavelengths of light. Recall that
E=Nhc/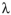 , where N=6.022X1023molecules/mol, h=1.583x10-37 kcal•s/molecule, and c=3.00x 1017nm/s.
, where N=6.022X1023molecules/mol, h=1.583x10-37 kcal•s/molecule, and c=3.00x 1017nm/s.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q46: Which carbon in the molecule below will
Q47: Predict the splitting pattern of the indicated
Q48: Match the three compounds shown here to
Q49: Match each of these 1H
Q50: Explain why chemical shifts are reported in
Q52: Which carbon in this molecule will have
Q53: If a solution with an absorbance of
Q54: The indole side chain of tryptophan (the
Q55: A compound with four degrees of unsaturation
Q56: Which of the following compounds corresponds to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents