The following transformation was carried out using one enantiomer of diethyl tartrate and the other reagents of the Sharpless asymmetric epoxidation reaction.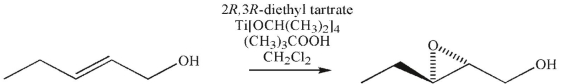
Predict the product that would result using the other enantiomer of diethyl tartrate.
Correct Answer:
Verified
Q46: Draw the structure of the most stable
Q47: Predict the product of the following transformation,
Q48: Draw the product of the following transformation:
Q49: Draw a mechanism for the following transformation.Show
Q50: Devise a multistep synthesis of the target
Q52: The molecule shown was the exclusive product
Q53: Draw the structure of the enol intermediate
Q54: Provide two different precursors and the reagents
Q55: Provide the missing reagents and structures.
Q56: Explain the difference between a heterogeneous catalyst
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents