When a sample of diethyl ether is allowed to stand, over time it reacts with molecular
oxygen to form peroxides. 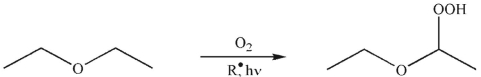
Propose an arrow-pushing mechanism to illustrate the formation of the peroxide from the ether starting material. Use 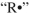 as the initiator. Show all curved arrows and single electrons.
as the initiator. Show all curved arrows and single electrons.
Correct Answer:
Verified
Q23: Predict the product of the following reaction.
Q24: Which of the following is a propagation
Q25: Predict the product of the following reaction.
Q26: Draw an arrow-pushing mechanism for the transformation
Q27: What would be expected to be the
Q29: Which of the following monobrominated products forms
Q30: Which of the following compounds is the
Q31: Predict the most likely products of β
Q32: Which of the following statements about rearrangements
Q33: What is the stereochemical outcome of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents