Anti-Markovnikov addition of HX to an alkene only works with HBr in the presence of peroxides
as a radical initiator.Use the bond dissociation data provided to explain why the reaction fails
with HCl and HI. 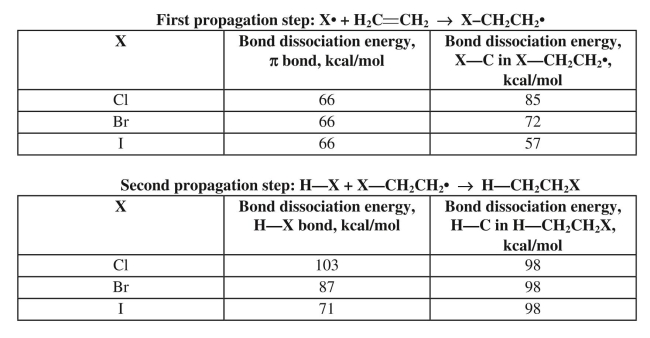
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q42: S-6-Bromo-1,6-dimethylcyclohexene reacts with hydrogen bromide in the
Q43: Devise a multistep synthesis of the target
Q44: A chemist would like to prepare a
Q45: Draw the two organic products formed from
Q46: Draw the stepwise mechanism for the free
Q48: What is the principal monobrominated product that
Q49: Provide an arrow-pushing mechanism for the formation
Q50: Predict the product of the following reaction.
Q51: Draw an arrow-pushing mechanism to show the
Q52: Using the bond dissociation energies provided below,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents