In the rearrangement of 2-butyne to 1-butyne, what causes the reaction to go to completion?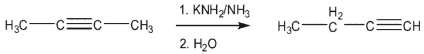
A) the increased stability of the terminal triple bond in the product
B) the high pKa of ammonia versus the methyl group in 2-butyne
C) the decreased activation energy for conversion of the allene intermediate to 1 -butyne
D) the much lower pKa of the acetylenic proton in the product versus the other species
E) the increased stability of the 1,2 -butadiene intermediate versus 2 -butyne
Correct Answer:
Verified
Q11: Rank these dienes in order of increasing
Q12: Which of the structures shown is in
Q13: Which of the following structures is the
Q14: Which of these structures is a conjugated
Q15: Which of these alkynes will not isomerize
Q17: Which of these structures is the product
Q18: Which of these compounds can exist as
Q19: Rank the following C4H6 isomers in
Q20: Which of the following structures is chiral?
Q21: Draw a mechanism for the formation of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents