When 1,3 -butadiene reacts with chlorine, a 50/50 mixture of 3,4 -dichloro-1-butene and (E) -1,4-dichloro-2-butene is generated. When the same diene is reacted with bromine, the 3,4 -dibromo-1-butene is the predominant product.. What could explain the difference in regioselectivity?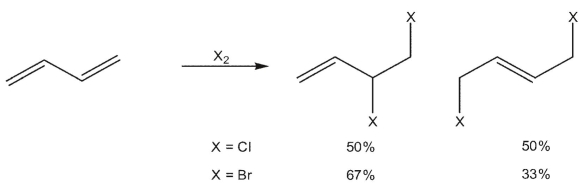
A) The more stable intermediate bromonium ion favors 1,2 -addition.
B) The more reactive bromide anion favors the kinetic product.
C) The more reactive chloride anion favors the thermodynamic product.
D) The more stable intermediate chloronium ion favors the 1,4 -addition product.
E) The electronegative bromine destabilizes the positive charge on the β-carbon.
Correct Answer:
Verified
Q1: Which of the following structures is a
Q2: From left to right, what is the
Q3: Which of the following compounds is the
Q5: Which of the following molecules contains the
Q6: How many nodes are there in the
Q7: Which of the following statements about the
Q8: Which of these statements about thermodynamic and
Q9: Which structure contains the longest C-C bond?
Q10: Which of the structures shown is in
Q11: Rank these dienes in order of increasing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents