Which of the following compounds has at least one lone pair in an sp2 orbital?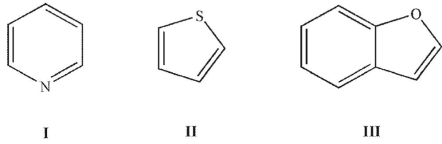
A) I
B) I and II
C) I and III
D) II and III
E) I, II, and III
Correct Answer:
Verified
Q18: Which is a Dewar resonance form of
Q19: Which of these structures is not aromatic?
Q20: Which of the following ions is not
Q21: Is the cis-CH2=CH-CH=CH-CH=CH2 molecule aromatic? Explain why
Q22: Compounds W, X, Y and Z are
Q24: Which of the following structures is the
Q25: Which of the following will not react
Q26: There are five resonance structures of 1,2-dimethoxycyclopropenyl
Q27: How many isomeric trisubstituted benzenes C3H3XYZ are
Q28: Which of these structures is the major
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents