Why is one equivalent of AlCl3 required for the transformation shown here?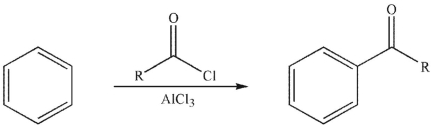
A) Either the oxygen or the chlorine in the acyl chloride can attach to the Al atom in AlCl3.
B) AlCl3 is needed to produce the acyl chloride during the course of the reaction.
C) The product forms a complex with the AlCl3.
D) The product would rearrange unless there were extra. AlCl3 present.
E) Both benzene and the acyl chloride form complexes with the AlCl3.
Correct Answer:
Verified
Q2: Which reagent would you use to accomplish
Q3: Which conditions would you use to accomplish
Q4: What is the electrophile in this reaction?
Q5: Which of the following statements is false?
A)Hydrogenation
Q6: What is the proper description of the
Q7: What is the electrophile in this reaction?
Q8: Which of the following compounds is activated
Q9: Which of the following would not react
Q10: What is the electrophile in this reaction?
Q11: Which of the following statements is true?
A)All
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents