Shown below is an electrophilic aromatic substitution reaction. Which of the following statements about this reaction is/are true?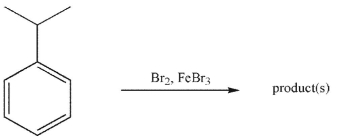
I. The products of ortho and para substitution are favored in the overall reaction.
II. The product of meta substitution is favored in the overall reaction.
III. For all three substitution pathways (ortho, meta, and para) the transition state in the rate-limiting step is lower in energy than the transition state for rate-limiting step in the bromination of benzene.
IV. Compared to the energy of the transition state for the rate-limiting step in the bromination of benzene, the transition state in the rate-limiting step for this reaction is lower in energy for the ortho and para pathways, but higher in energy for the meta pathway.
A) I
B) II
C) I and III
D) I and IV
E) II and IV
Correct Answer:
Verified
Q26: Draw the structure of the electrophile in
Q27: Draw an energy diagram for the chlorination
Q28: Bromination of aniline results in 2,4,6
Q29: Which of the following compounds will undergo
Q30: Which of the following compounds is the
Q32: Draw a mechanism for the transformation shown
Q33: Predict the product of the following reaction.
Q34: Which of the following compounds is the
Q35: Draw a mechanism to illustrate the following
Q36: Predict the product of the following reaction.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents