Phenol usually reacts easily with electrophiles.However, nitration of phenol with nitric acid is unsuccessful since the oxidation of phenol to p-quinone is faster than the electrophilic substitution. 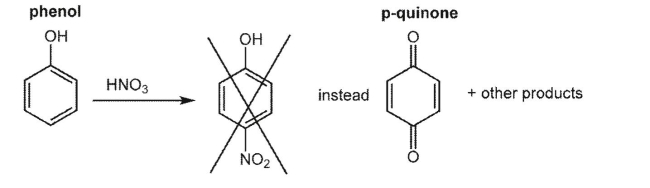 However, the nitration reaction shown below is successful. Explain why the reactivity of starting material is different from that of phenol. Draw the nitration product.
However, the nitration reaction shown below is successful. Explain why the reactivity of starting material is different from that of phenol. Draw the nitration product. 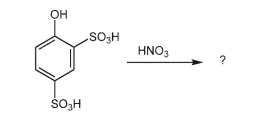
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q41: What conditions are required to synthesize the
Q42: What is the product of the following
Q43: Aniline is more activating towards electrophilic aromatic
Q44: Design a multistep synthesis for the following
Q45: A chemist attempted the following Friedel-Crafts alkylation
Q47: Predict the product of the following reaction
Q48: Draw a mechanism for the following transformation.Include
Q49: Design a multistep synthesis for the transformation
Q50: Predict the major organic product and draw
Q51: Draw a mechanism to illustrate the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents