For compound A, in acid, it is the hydroxyl oxygen that is protonated to a greater extent than the
carbonyl oxygen. 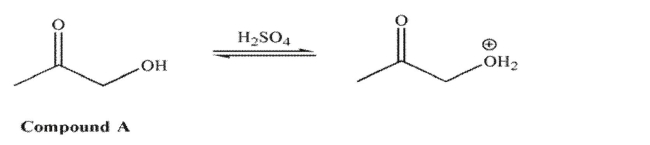 However, for compound B in acid, it is the carbonyl oxygen that is protonated to a greater extent
However, for compound B in acid, it is the carbonyl oxygen that is protonated to a greater extent
than the hydroxyl oxygen. 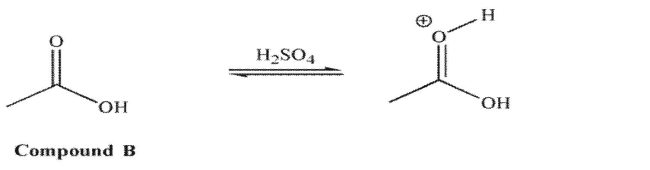 Explain the difference in these results.
Explain the difference in these results.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q26: Which of the following is the correct
Q27: What reagent would you use to accomplish
Q28: What is the product of the following
Q29: Which has more resonance stabilization, an ester
Q30: Why is acidic amide hydrolysis irreversible?
A)The amine
Q32: Draw the structure of methyl 3,3-dimethylbutanoate.
Q33: Which of the following compounds will not
Q34: What conditions could be used to accomplish
Q35: Provide an acceptable name for the structure
Q36: What is the final product of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents