For the following reaction: 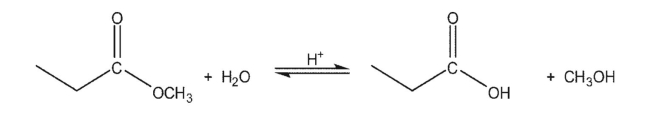 How would each of the following affect the position of the equilibrium?
How would each of the following affect the position of the equilibrium?
A)Running the reaction in methanol as a solvent
B)Running the reaction in a still at a temperature of 85-90 °C
C)Running the reaction in the presence of anhydrous sodium sulfate at room temperature
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q40: Which of the following is an isolable
Q41: Match the compound shown to the correct
Q42: Predict the product of the following reaction
Q43: List the reagents required to convert benzoyl
Q44: Draw a mechanism for the following transformation.Include
Q46: Draw the structure of the compound with
Q47: Predict the major organic product of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents