Why does the following reaction result in a racemic mixture of products? 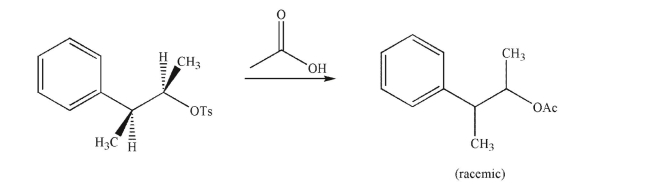
A) The acetic acid reagent is achiral.
B) Once the tosylate ionizes, it leaves a planar carbocation that can be attacked with equal likelihood at either side.
C) The key intermediate is achiral.
D) Both the enantiomeric products are equal in energy.
E) Neighboring group participation diminishes the selectivity of the reaction.
Correct Answer:
Verified
Q1: What reagent(s) could be used to perform
Q2: Consider these two similar reactions and their
Q3: What experimental observation rules out the possibility
Q4: The following solvolysis reaction goes through a
Q5: Anchimeric assistance
A)generally slows the rate of a
Q7: Which product would be expected to form
Q8: Which of the following bicyclic amines will
Q9: Predict the product of the reaction conditions
Q10: Which of the following is an intermediate
Q11: Which of these can participate as neighboring
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents