Neighboring Group Effects Are More Strongly Observed in Trans-2-Hydroxycyclopentylsulfonates (Structure
Neighboring group effects are more strongly observed in trans-2-hydroxycyclopentylsulfonates (Structure A ) than in the corresponding nitroarenesulfonates (Structure B).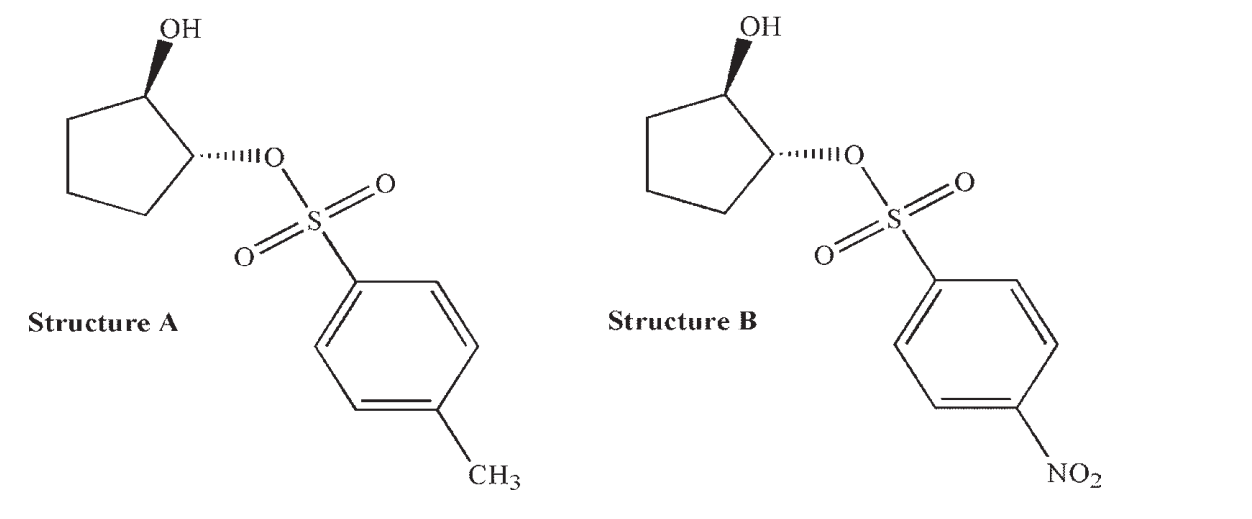
Draw the product you would expect from the reaction of structure A with an acetate ion, and propose an explanation why a neighboring group effect may not be as significant for structure B.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q22: Explain why trans-1-chloro-2-phenylcyclohexane A would be expected
Q23: Structure A reacts faster with nucleophiles than
Q24: A carbene in the singlet state is
Q25: Explain why compound A below would be
Q26: Use the idea of neighboring group participation
Q28: How many different 13C NMR signals
Q29: Draw the mechanism of the following reaction;
Q30: Predict the product and draw a mechanism
Q31: The syn-tosylate of 7-hydroxynorbornene undergoes hydrolysis 104
Q32: Propose a mechanism for the following transformation,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents