Explain why trans-1-chloro-2-phenylcyclohexane A would be expected to undergo hydrolysis in acetic acid much more rapidly than the corresponding cis-isomer B :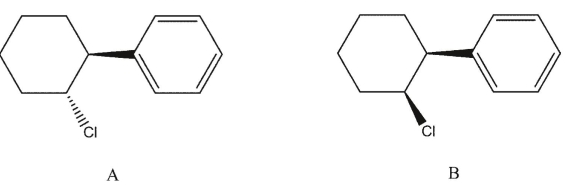
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q17: Which of the following reactions would not
Q18: Coates’ cation
A) is best described as a
Q19: Which statement best summarizes the stereochemical outcomes
Q20: Which of the following structures have neighboring
Q21: Propose an explanation for the difference in
Q23: Structure A reacts faster with nucleophiles than
Q24: A carbene in the singlet state is
Q25: Explain why compound A below would be
Q26: Use the idea of neighboring group participation
Q27: Neighboring group effects are more strongly observed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents