After careful ethical review of planned procedures, a researcher tests a new brain activation drug by taking a sample of 50 volunteers and randomly assigning 25 participants to the treatment condition and 25 to the control (placebo) condition.She then carries out an fMRI scan to assess the level of activation in regions of the participants' brains 30 minutes after administering the drug.She carries out a between-participants t-test in SPSS which provides the output shown below: 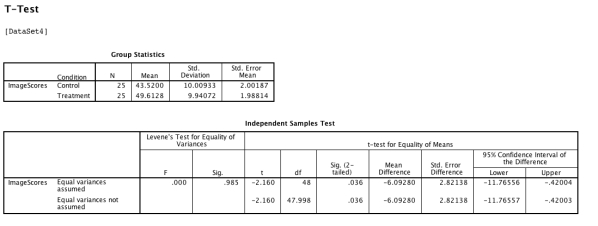 Which of the following statements is true?
Which of the following statements is true?
A) The result is not significant because the p value is .985.
B) The result is not significant because a negative t-test indicates a negative result.
C) There are 48 degree of freedom because N is 50 and there are two groups.
D) The scan measures processes within-participants' brains so the researcher should have performed a within-participants t-test.
E) We need to make sure the correlation between the control and treatment scores is more than .3 before we can confidently use the t-test.
Correct Answer:
Verified
Q15: "An estimated range of values for a
Q16: "The hypothesis that the research reveals no
Q17: "The largest probability that the researcher is
Q18: "The hypothetical distribution of expected differences between
Q19: After careful ethical review of planned procedures,
Q21: If a researcher is concened that the
Q22: A personality researcher revisits the hypothesis that
Q23: A personality researcher revisits the hypothesis that
Q24: "A test with two rejection regions that
Q25: "The conditions that must be satisfied before
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents