After careful ethical review of planned procedures, a researcher tests a new brain activation drug by taking a sample of 50 volunteers and randomly assigning 25 participants to the treatment condition and 25 to the control (placebo) condition.She then carries out an fMRI scan to assess the level of activation in regions of the participants' brains 30 minutes after administering the drug.She carries out a between-participants t-test in SPSS which provides the output shown below: 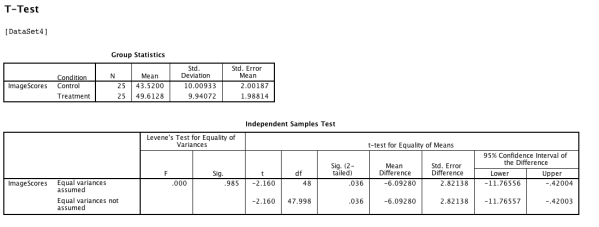 What further advice is appropriate to give the researcher here?
What further advice is appropriate to give the researcher here?
A) The variances look as if they could be unequal.
B) It is worth ensuring that the distributions of the scores are normal.
C) The negative confidence interval suggests she needs to reduce statistical uncertainty further.
D) The t-test should be accompanied with a measure of effect size such as Cohen's d.
E) Both (a) and (d) .
Correct Answer:
Verified
Q1: If an experimenter conducts a t-test to
Q2: A clinical neuropsychologist develops a new treatment
Q3: A psychology professor has set a test
Q5: Which of the following does not compromise
Q6: If an experimenter conducts a t-test to
Q7: If an experimenter conducts a t-test to
Q8: A clinical neuropsychologist develops a new treatment
Q9: "The hypothetical distribution of differences between the
Q10: After careful ethical review of planned procedures,
Q11: A clinical neuropsychologist develops a new treatment
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents