Multiple Choice
Calculate ΔS° for the reaction 4Cr(s) + 3O2(g) → 2Cr2O3(s) 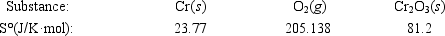
A) −548.1 J/K
B) −147.7 J/K
C) 147.7 J/K
D) 310.1 J/K
E) 548.1 J/K
Correct Answer:
Verified
Related Questions
Q54: The second law of thermodynamics tells us
Q55: Elemental boron can be formed by reaction
Q56: A certain process has ΔH° > 0,
Q57: In order for a process to be
Q58: A sample of water is heated at
Q60: Given: H2O(l) → H2O(g) ΔH° = 40.7
Q61: "A diamond is forever" is one of
Q62: Use the thermodynamic data at 298 K
Q63: Use the given data at 298 K
Q64: What is the free energy change, ΔG°,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents