Calculate ΔS° for the combustion of propane. C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) 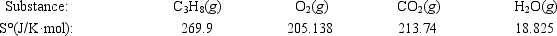
A) −100.9 J/K
B) −72.5 J/K
C) 72.5 J/K
D) 100.9 J/K
E) 877.5 J/K
Correct Answer:
Verified
Q39: Which relationship or statement best describes ΔS°
Q40: In which one of the following pairs
Q41: For a process with ΔS < 0,
Q42: Which one of the following changes of
Q43: Consider the following quantities used in thermodynamics:
Q45: Given: H2O(l) → H2O(s) ΔH° = −6.02
Q46: Calculate ΔS° for the reaction 2Cl2(g) +
Q47: Calculate ΔS° for the reaction SiCl4(g) +
Q48: For a chemical reaction to be spontaneous
Q49: For a chemical reaction to be non-spontaneous
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents