Write the mass-action expression, Qc, for the following chemical reaction. NO(g) + ½Br2(g) ⇄ NOBr(g)
A) 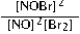
B) 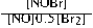
C) 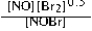
D) 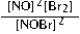
E) 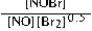
Correct Answer:
Verified
Q35: What is the mass-action expression, Qp, for
Q36: What is the mass-action expression, Qc, for
Q37: Carbon monoxide and chlorine combine in an
Q38: When a chemical system is at equilibrium,
A)the
Q39: Write the mass-action expression, Qc, for the
Q41: Consider the equilibrium reaction: H2(g) + Br2(g)
Q42: The equilibrium constant for the reaction of
Q43: About half of the sodium carbonate produced
Q44: At 500°C the equilibrium constant, Kp, is
Q45: The equilibrium constant for reaction (1) below
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents