Identify the particle emitted by the nucleus that undergoes the transformation shown in the figure. 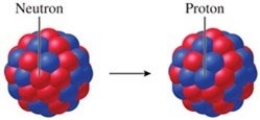
A) an alpha particle
B) a beta particle (electron)
C) a positron
D) a gamma ray
E) a neutron
Correct Answer:
Verified
Q27: Bombardment of boron-10 with a projectile particle
Q28: Uranium-238 decays by alpha emission to form
Q29: Radon-222 decays by alpha emission to form
Q30: Predict the type of radiation emitted when
Q31: What is the missing symbol in the
Q33: Bombardment of boron-10 with a neutron produces
Q34: Carbon-14 decays by beta emission to form
Q35: Protactinium-234 decays by beta emission to form
Q36: Potassium-40 decays by beta emission to form
Q37: What is the missing symbol in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents