What type of radioactive decay would be expected for the unstable nuclide 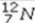 ?
?
A) alpha particle emission
B) beta particle (electron) emission
C) positron emission
D) gamma ray emission
E) neutron emission
Correct Answer:
Verified
Q37: What is the missing symbol in the
Q38: The neutron was discovered in 1932 by
Q39: Thorium-234 decays by beta emission to form
Q40: Uranium-234 decays by alpha emission to form
Q41: Strontium-90 has a half-life of 28 years.
Q43: Select the most stable isotope from the
Q44: The half-life of technetium-99 is 6.0 hours.
Q45: The half-life of technetium-99 is 6.0 hours.
Q46: Select the most stable isotope from the
Q47: The half-life of iodine-123 is 13.3 hours.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents