Consider a voltaic cell that corresponds to the following reaction: Cu(s) + 2Ag+(aq) → Cu2+(aq) + Ag(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 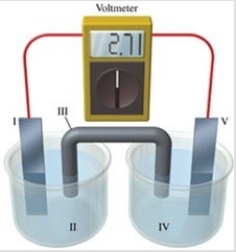
A) I is the copper electrode, which is the anode.
B) II is the Ag+ solution.
C) III is the salt bridge.
D) V contains the substance that is being reduced.
E) V is the silver electrode, which is the cathode.
Correct Answer:
Verified
Q44: Consider the reaction: Cu(s)+ 4HNO3(aq)→ Cu(NO3)2(aq)+ 2NO2(g)+
Q45: For the following reaction, what is the
Q46: Consider the reaction: Zn(s)+ H2SO4(aq)→ ZnSO4(aq)+ H2(g)Which
Q47: Consider the reaction: Cu(s)+ 4HNO3(aq)→ Cu(NO3)2(aq)+ 2NO2(g)+
Q48: Consider a voltaic cell that corresponds to
Q50: Consider a voltaic cell that corresponds to
Q51: Consider the reaction: Sn2+(aq)+ 2Fe3+(aq)→ Sn4+(aq)+ 2Fe2+(aq)Which
Q52: In a voltaic cell, the electron flow
Q53: Consider the reaction: Zn(s)+ H2SO4(aq)→ ZnSO4(aq)+ H2(g)Which
Q54: Consider the reaction: Sn2+(aq)+ 2Fe3+(aq)→ Sn4+(aq)+ 2Fe2+(aq)Which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents