Consider a voltaic cell that corresponds to the following reaction: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 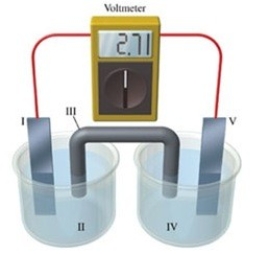
A) I is the zinc electrode, which is the anode.
B) II is the Cu2+ solution.
C) III is the salt bridge.
D) IV contains the substance that is being reduced.
E) V is the copper electrode, which is the cathode.
Correct Answer:
Verified
Q37: Consider the following oxidation-reduction reaction: 2Fe3+(aq)+ 2Hg(l)+
Q38: Consider the reaction: 2HgO(s)→ 2Hg(l)+ O2(g)Which of
Q39: Consider the reaction: Ba(NO3)2(aq)+ Na2SO4(aq)→ BaSO4(s)+ 2NaNO3(aq)Which
Q40: What is the oxidation number of sulfur
Q41: Consider a voltaic cell that corresponds to
Q43: Consider the reaction: Sn2+(aq)+ 2Fe3+(aq)→ Sn4+(aq)+ 2Fe2+(aq)Which
Q44: Consider the reaction: Cu(s)+ 4HNO3(aq)→ Cu(NO3)2(aq)+ 2NO2(g)+
Q45: For the following reaction, what is the
Q46: Consider the reaction: Zn(s)+ H2SO4(aq)→ ZnSO4(aq)+ H2(g)Which
Q47: Consider the reaction: Cu(s)+ 4HNO3(aq)→ Cu(NO3)2(aq)+ 2NO2(g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents