The figure shows the electrolysis of molten AlCl3. What reaction occurs at the anode of this cell? 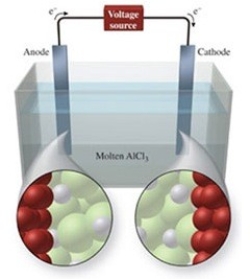
A) AlCl3(l) → AlCl2(l)
B) Cl-(l) + e- → Cl2-
C) Al3+(l) + 3e- → Al(l)
D) 2Cl-(l) + 2e- → Cl2(g)
E) 2Cl-(l) → Cl2(g) + 2e-
Correct Answer:
Verified
Q90: Given the following information about the activity
Q91: Given the following information about the activity
Q92: Consider the reaction: Zn(s)+ NO3−(aq)→ NH3(aq)+ Zn(OH)42−(aq)When
Q93: The products of the electrolysis of molten
Q94: The figure shows the electrolysis of molten
Q96: Given the following information about the activity
Q97: Consider the reaction: H3AsO3(aq)+ BiO3−(aq)→ H3AsO4(aq)+ Bi(s)When
Q98: Consider the half-reaction NH4+(aq)→ NO3-(aq). When the
Q99: Consider the half-reaction NO3−(aq)→ NO(aq). When the
Q100: The figure shows the electrolysis of molten
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents