The figure shows the electrolysis of molten AlCl3. What is the balanced equation for the reaction? 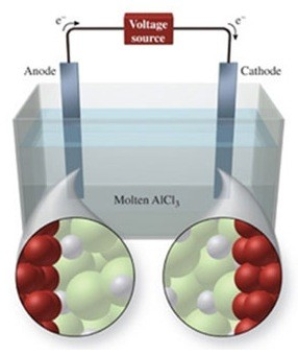
A) AlCl3(l) → Al3+(l) + 3Cl−(l)
B) AlCl3(l) → Al(l) + Cl3(g)
C) 2AlCl3(l) → 2Al(l) + 3Cl2(g)
D) AlCl3(s) → Al3+(aq) + 3Cl−(aq)
E) 2AlCl3(s) → 2Al(s) + 3Cl2(s)
Correct Answer:
Verified
Q79: The following reaction occurs in a lead
Q80: Consider the skeletal equation: Sn2+(aq)+ Fe3+(aq)→ Sn4+(aq)+
Q81: Consider the half-reaction O2(aq)→ OH-(aq). When the
Q82: The figure shows the electrolysis of molten
Q83: The figure shows the electrolysis of molten
Q85: Consider the reaction: Al(s)+ H2O(l)→ Al(OH)4−(aq)+ H2(g)When
Q86: Consider the reaction: CrO42−(aq)+ HSO3−(aq)→ Cr3+(aq)+ SO42−(aq)When
Q87: Given the following information about the activity
Q88: In any electrolytic cell, the cathode is
Q89: Consider the half-reaction ClO−(aq)→ Cl−(aq). When the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents