Consider the reaction and the value of its equilibrium constant: S2Cl2(g) + Cl2(g) ⇌ 2SCl2(g) Keq = 4 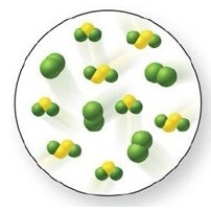 Examine the figure, and determine if the system is at equilibrium. If it is not, in which direction will it proceed to reach equilibrium?
Examine the figure, and determine if the system is at equilibrium. If it is not, in which direction will it proceed to reach equilibrium?
A) The reaction is at equilibrium.
B) The reaction is not at equilibrium; it will shift to the left.
C) The reaction is not at equilibrium; it will shift to the right.
D) It is not possible to tell if the reaction is at equilibrium.
E) To reach equilibrium, the value of Keq must change.
Correct Answer:
Verified
Q50: The value of the equilibrium constant for
Q51: Which of the following statements is correct
Q51: Which of the following statements is correct
Q52: The value of the equilibrium constant for
Q53: The value of the equilibrium constant for
Q54: The value of the equilibrium constant for
Q56: Which equilibrium constant represents a reaction that
Q57: The value of the equilibrium constant for
Q58: The value of the equilibrium constant for
Q60: Consider the following reaction at a specific
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents