The reaction S2Cl2(g) + Cl2(g) ⇌ 2SCl2(g) has an equilibrium constant of K = 8. The image shows a partially reacted system. What is the composition of this system when the reaction reaches equilibrium? 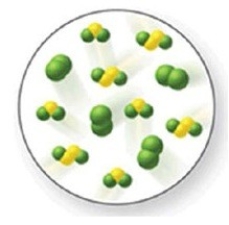
A) 1 S2Cl2, 3 Cl2, 10 SCl2
B) 2 S2Cl2, 4 Cl2, 8 SCl2
C) 3 S2Cl2, 5 Cl2, 6 SCl2
D) 4 S2Cl2, 6 Cl2, 4 SCl2
E) 5 S2Cl2, 7 Cl2, 2 SCl2
Correct Answer:
Verified
Q39: Consider the following reaction carried out in
Q40: The graph shows the change in concentrations
Q41: Which equilibrium constant represents a reaction that
Q42: Consider the reaction CO(g)+ H2O(g)⇌CO2(g)+ H2(g), represented
Q43: The value of the equilibrium constant for
Q45: Consider the following reaction at a specific
Q46: The value of the equilibrium constant for
Q47: Consider the following reaction at a specific
Q48: For a given reaction, which of the
Q49: Consider the following reaction at a specific
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents