Is the system shown in the figure at equilibrium? If not, in which direction must the reaction proceed? 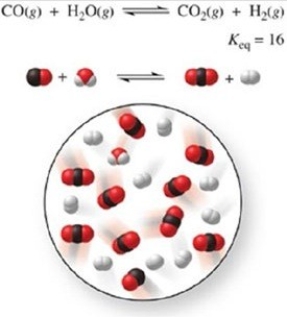
Correct Answer:
Verified
Q98: The high potential-energy chemical species that is
Q99: A catalyst increases the rate of a
Q100: An activated complex is a short-lived, high-energy
Q101: The value of the equilibrium constant for
Q102: Consider the reaction and its equilibrium constant:
Q104: Using collision theory, explain why reaction rates
Q105: If the initial concentrations of reactants and
Q106: If the equilibrium constant at 25°C is
Q107: What is the relationship between the following
Q108: When yeast bread dough is placed in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents