From the vapor pressure curve of acetone, it can be seen that the normal boiling point of acetone is about ________. 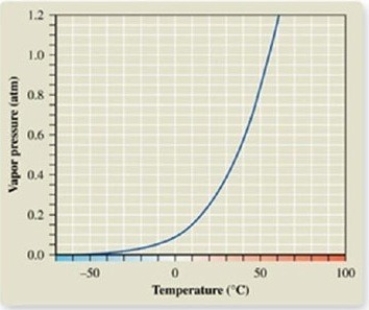
A) -50ºC
B) 0ºC
C) 50ºC
D) 55ºC
E) 100ºC
Correct Answer:
Verified
Q14: What phase transition is occurring between points
Q15: Three phases of water are shown in
Q16: What phase transition is occurring between points
Q17: What phase transition is occurring between points
Q18: What phase change is occurring in the
Q20: What phase transition is occurring between points
Q21: Calculate the amount of heat energy, in
Q22: Which of the following statements regarding intermolecular
Q23: The physical properties of a substance influenced
Q24: The diagram represents the physical state of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents