The atmospheric pressure on Mount Everest is about 0.30 atm. From the vapor pressure curve of propane, it can be seen the boiling point of propane at this elevation is about ________. 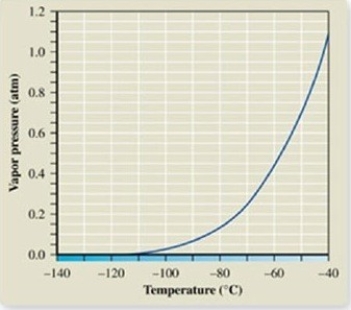
A) −137ºC
B) −120ºC
C) −67ºC
D) −42ºC
E) 0ºC
Correct Answer:
Verified
Q3: What phase change is occurring in the
Q4: The pictured change of state occurs at
Q5: The pictured change of state occurs at
Q6: Which of the following statements is correct?
A)At
Q7: From the vapor pressure curve of propane,
Q9: Which of the following statements is correct?
A)When
Q10: Which of the following statements is correct?
A)The
Q11: Which of the following statements is correct?
A)A
Q12: The pictured change of state occurs at
Q13: Which of the molecular-level images in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents