Of the two molecules shown in the figure, one would expect that CO2 has a higher boiling point than NO2 because CO2 is linear and nonpolar. 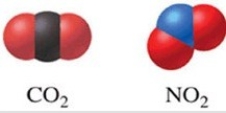
Correct Answer:
Verified
Q100: Identify the type of solid shown in
Q101: One would expect that water is more
Q102: Explain why a container of liquid can
Q103: If there were no intermolecular forces, most
Q104: Explain why different liquids have different vapor
Q106: Detergents are used to increase the surface
Q107: What is the boiling point in °C
Q108: One would expect that since CO is
Q109: Explain how a pressure cooker works by
Q110: What is the boiling point in °C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents