Consider the following Lewis structure for CO32-. How many resonance forms does this ion have? 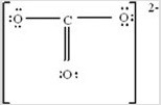
Correct Answer:
Verified
Q107: Which of the following molecules is polar?
A)CCl4
B)H2S
C)SO3
D)CO2
E)BeF2
Q108: Which of the molecules in the figure
Q109: Electronegativity is a measure of the amount
Q110: If a molecule has polar bonds, the
Q111: Resonance is a representation of how electrons
Q113: A trigonal planar molecule will have an
Q114: Draw a Lewis structure for H2S. How
Q115: Draw a Lewis structure for PH3. How
Q116: Which of the following molecules is polar?
A)BF3
B)CH4
C)CS2
D)PCl3
E)BeCl2
Q117: Diatomic elements such as F2 and Cl2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents