When the mixture of molecules shown in the molecular-level image undergoes complete reaction, all of these molecules are converted to products. Which of the following reactions could this represent? 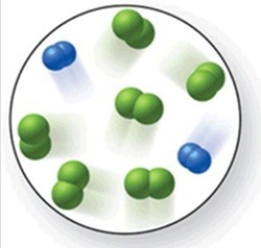
A) 2N2 + 3O2 → 2N2O3
B) N2 + 2Cl2 → N2Cl4
C) O2 + 2H2 → 2H2O
D) N2 + 3Cl2 → 2NCl3
E) 3N2 + 2H2 → 3N2H4
Correct Answer:
Verified
Q2: When potassium carbonate, K2CO3, dissolves in water,
Q3: Consider the reaction between acetylene, C2H2, and
Q4: Which of the following equations is balanced?
A)P4(s)+
Q5: Phosphine, PH3, a reactive and poisonous compound,
Q6: When one molecule of propane, C3H8, burns
Q8: When sodium sulfate, Na2SO4, dissolves in water,
Q9: When acetylene, C2H2, a fuel used in
Q10: Which of the following is the best
Q11: Which of the following is the best
Q12: When the mixture of molecules shown in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents