Consider the following specific heats of metals. 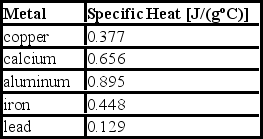 If the same amount of heat is added to 25.0 g of each of these metals, all at the same initial temperature, which metal will have the highest final temperature?
If the same amount of heat is added to 25.0 g of each of these metals, all at the same initial temperature, which metal will have the highest final temperature?
A) copper
B) calcium
C) aluminum
D) iron
E) lead
Correct Answer:
Verified
Q84: Iron metal reacts with hydrochloric acid as
Q85: A 43 g serving of a chocolate
Q86: Iron metal reacts with hydrochloric acid as
Q87: Which of the following processes is exothermic?
A)boiling
Q88: If 75.0 J of heat energy is
Q90: Consider the following specific heats of metals.
Q91: The following reaction absorbs 393 kJ of
Q92: When carbon dioxide is formed from its
Q93: An equal quantity of heat is transferred
Q94: Which of the following processes is exothermic?
A)ice
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents