The figure shows a reaction between methane gas (natural gas, CH4) and oxygen to produce carbon dioxide and water. Is the diagram accurate, and if not, what is wrong with it, and how could it be fixed? 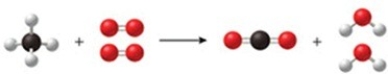
A) There are too many hydrogen atoms on the right-hand side of the reaction arrow. Remove one hydrogen atom from each water molecule.
B) There are too many oxygen atoms on the right-hand side of the reaction arrow. Remove one oxygen atom from the carbon dioxide.
C) The diagram is accurate as shown.
D) There are not enough carbon atoms on the left-hand side of the reaction arrow. Add another methane molecule on the left, and then another carbon dioxide molecule on the right of the arrow.
E) There are not enough oxygen molecules on the left-hand side of the reaction arrow. Add another oxygen molecule on the left of the arrow, and another water molecule on the right.
Correct Answer:
Verified
Q1: Zinc metal will react with aqueous hydrochloric
Q2: Consider the following chemical equations. Select the
Q3: In the figure shown, is a chemical
Q5: Consider the following chemical equations. Select the
Q6: In the figure shown, is a chemical
Q7: When solid ammonium carbonate is heated, it
Q8: Gaseous nitrogen monoxide reacts with oxygen gas
Q9: Fireworks which give off bright flashes of
Q10: The figure shows the chemical reaction between
Q11: Xenon gas reacts with fluorine gas to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents