Sulfur dioxide gas reacts with oxygen gas to form sulfur trioxide gas. Based on the initial reaction mixture, what should be present after the reaction occurs? 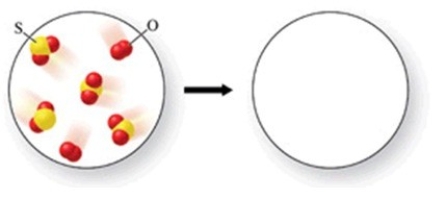
A) 2 molecules of sulfur trioxide, and 2 molecules of sulfur dioxide
B) 4 molecules of sulfur trioxide, and 1 molecule of oxygen
C) 3 molecules of sulfur trioxide, and 1 molecule of oxygen
D) 3 molecules of sulfur trioxide, and 1 molecule of sulfur dioxide
E) 4 molecules of sulfur trioxide
Correct Answer:
Verified
Q15: In the figure shown, is a chemical
Q16: Which of the following changes represents a
Q17: The figure shows a reaction between xenon
Q18: The figure shows a reaction between hydrogen
Q19: Consider the following chemical equations. Select the
Q21: The gases carbon dioxide and hydrogen can
Q22: Which of the following is a balanced
Q23: Balance the following skeletal equation: Ba(NO3)2(aq)+ K2SO4(aq)→
Q24: Balance the following skeletal equation: NH3(g)+ O2(g)→
Q25: After the following equation is properly balanced,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents