Which of the following is a balanced equation with lowest whole-number coefficients that represents the reaction shown in the figure? 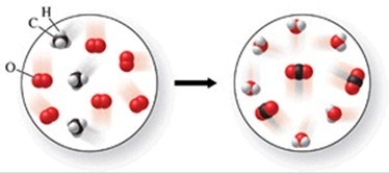
A) 2CH3 + 3O2 → 2CO2 + 3H2O
B) CH4 + 2O2 → CO2 + 2H2O
C) 3CH3 + 6O2 → 3CO2 + 6H2O
D) 3CH4 + 5O2 → 3CO2 + 5H2O
E) CH4 + O2 → CO + H2O
Correct Answer:
Verified
Q34: A reaction which has two compounds as
Q35: Balance the following skeletal equation: Pb(NO3)2(aq)+ KI(aq)→
Q36: A reaction which has one element and
Q37: A reaction which has one compound as
Q38: Balance the following skeletal equation: C2H5OH(l)+ O2(g)→
Q40: Balance the following skeletal equation: HCl(g)+ O2(g)→
Q41: Which of the following is a balanced
Q42: When aqueous solutions of H2SO4 and NaOH
Q43: The class of the reaction shown in
Q44: What is the correct formula for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents