Methane burns with oxygen gas to form carbon dioxide and water vapor. The class of this reaction is 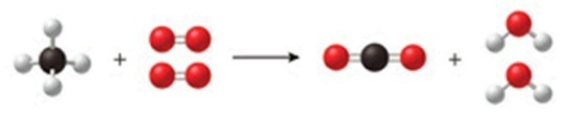
A) double-displacement reaction.
B) decomposition reaction.
C) combustion reaction.
D) combination reaction.
E) single-displacement reaction.
Correct Answer:
Verified
Q41: Which of the following is a balanced
Q42: When aqueous solutions of H2SO4 and NaOH
Q43: The class of the reaction shown in
Q44: What is the correct formula for the
Q45: A piece of magnesium metal gradually forms
Q47: Crystals of red mercury(II)oxide, when heated, form
Q48: A piece of magnesium metal is placed
Q49: Which of the following equations best describes
Q50: What are the products of the combustion
Q51: What is the product of the combination
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents