The class of the reaction shown in the figure is 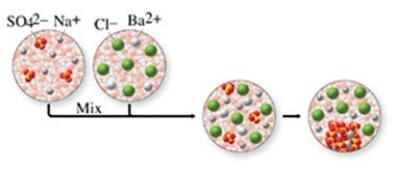
A) combination reaction.
B) combustion reaction.
C) single-displacement reaction.
D) double-displacement reaction.
E) decomposition reaction.
Correct Answer:
Verified
Q50: What are the products of the combustion
Q51: What is the product of the combination
Q52: Which of the following is a balanced
Q53: Sodium metal reacts with water in a
Q54: Which of the following is a balanced
Q56: Classify the following reaction: 2C8H18(l)+ 25O2(g)→ 16CO2(g)+
Q57: The class of the reaction shown in
Q58: Which of the following is a balanced
Q59: The class of the reaction shown in
Q60: When heated, calcium carbonate (limestone)undergoes a decomposition
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents