Rank the substances in the figure from least atoms per mole to most atoms per mole. 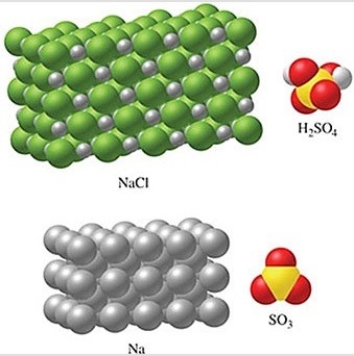
A) SO3 < H2SO4 < NaCl < Na
B) Na < NaCl < SO3 < H2SO4
C) Na < NaCl < H2SO4 < SO3
D) NaCl < Na < SO3 < H2SO4
E) H2SO4 < SO3 < NaCl < Na
Correct Answer:
Verified
Q35: Calculate the molar mass of Fe3(PO4)2.
A)237.64 g/mol
B)262.52
Q36: Calculate the molar mass of C3H6Cl2.
A)155.08 g/mol
B)77.54
Q37: The formula for novocain, a local anesthetic,
Q38: Rank the substances in the figure from
Q39: If 7.50 × 1024 molecules of a
Q41: Rank the following elements in order from
Q42: If the molar mass of a substance
Q43: If the molar mass of a substance
Q44: Rank the following elements in order from
Q45: A 50.0 g sample of a compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents