Which of the molecules in the figure have an empirical formula that is different from their molecular formula? 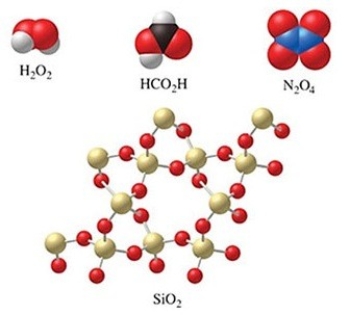
A) H2O2, N2O4, and HCO2H
B) SiO2
C) H2O2 and N2O4
D) all of the compounds
E) none of the compounds
Correct Answer:
Verified
Q79: What is the mass of 4.5 ×
Q80: What is the mass of 7.4 ×
Q81: Given the following molecular formulas, determine the
Q82: The following minerals contain lead. Rank the
Q83: How much water had to be added
Q85: Butyric acid has a very unpleasant odor.
Q86: Which of the substances in the figure
Q87: Given the following molecular formulas, determine the
Q88: Which of the following statements regarding empirical
Q89: Acetaminophen is the active ingredient in Tylenol.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents