Of the substances shown in the figure, only N2O3 has an empirical formula that is the same as its molecular formula. 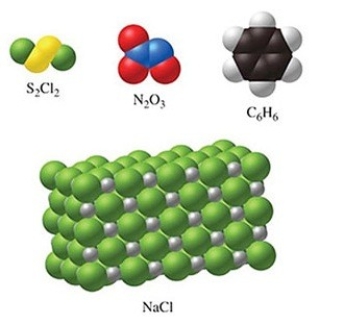
Correct Answer:
Verified
Q102: If 100.0 mL of 0.426 M NaOH
Q103: A formula written with the simplest ratio
Q104: What mass of NaCl is present in
Q105: Calculate the volume of 12.0 M HCl
Q106: Image B in the figure shows a
Q108: Calculate the molarity of a solution consisting
Q109: One mole of methane (CH4)and one mole
Q110: How much water must be added to
Q111: Calculate the moles and the mass of
Q112: The substance in the figure with the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents