The isotope symbol for an ion that has 13 protons, 14 neutrons, and 10 electrons is:
A) 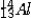
B) 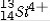
C) 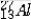
D) 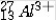
E) none of these
Correct Answer:
Verified
Q55: Which of the following statements does not
Q56: Sodium reacts vigorously with water to form
Q57: Which one of the following has more
Q58: Boron has two isotopes: B-10 and B-11,
Q59: Which of the following statements regarding relative
Q61: Which of the following is the most
Q62: To the correct number of significant figures,
Q63: To the correct number of significant figures,
Q64: When comparing 1000 amu of carbon atoms
Q65: Elements in Group IIA (2)are called:
A)halogens.
B)noble gases.
C)alkali
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents