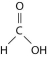
-The illustration above shows a representation of formic acid. A formic acid molecule
A) will form hydrogen bonds with water molecules.
B) has a tetrahedral configuration of hybrid electron orbitals for the carbon atom.
C) consists of largely nonpolar covalent bonds.
D) is held together by hydrogen bonds.
E) has a tetrahedral shape and will form hydrogen bonds with water molecules.
Correct Answer:
Verified
Q56: Q57: Van der Waals interactions result when Q58: Which of these systems is least likely Q59: Which of the following explains most specifically Q60: Q62: Q63: Which one of the atoms shown would Q64: Which one of the atoms shown would Q65: Q66: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
A) hybrid![]()
![]()
![]()
![]()

