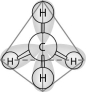
-In the methane molecule shown in the figure above, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electron orbitals in these bonds are said to be
A) double orbitals.
B) tetrahedral orbitals.
C) complex orbitals.
D) hybrid orbitals.
E) polar orbitals.
Correct Answer:
Verified
Q66: Q67: Q68: Which statement is true of all atoms Q69: Q70: The reactivity of an atom arises from Q72: Which of the following pairs of atoms Q73: In the term trace element, the modifier Q74: What is the atomic number of the Q75: A group of molecular biologists is trying Q76: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
A)![]()

