The following questions are based on the reaction A + B ↔ C + D shown in Figure 8.1.
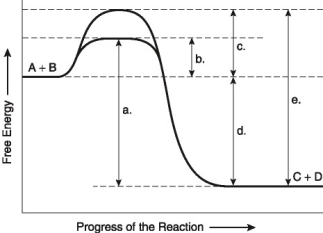
Figure 8.1
-Assume that the reaction in Figure 8.1 has a ΔG of -5.6 kcal/mol. Which of the following would be true?
A) The reaction could be coupled to power an endergonic reaction with a ΔG of +6.2 kcal/mol.
B) The reaction could be coupled to power an exergonic reaction with a ΔG of +8.8 kcal/mol.
C) The reaction would result in a decrease in entropy (S) and an increase in the total energy content (H) of the system.
D) The reaction would result in an increase in entropy (S) and a decrease in the total energy content (H) of the system.
E) The reaction would result in products (C + D) with a greater free-energy content than in the initial reactants (A + B) .
Correct Answer:
Verified
Q67: The following questions are based on the
Q68: The following questions are from the end-of-chapter
Q69: The following questions are based on the
Q70: The following questions are based on the
Q71: The following questions are from the end-of-chapter
Q73: The following questions are based on the
Q74: The following questions are based on the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents