The following is an example of a first-order reaction involving the _____. 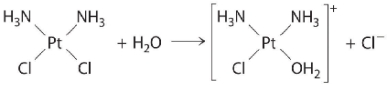
A) reaction of t-butyl bromide with water to give t-butanol
B) hydrolysis of the anticancer drug cisplatin
C) oxidation of ethanol to acetaldehyde in the liver
D) decomposition of N2O on a platinum surface
E) hydrolysis of aspirin
Correct Answer:
Verified
Q20: Distinguish between differential and integrated rate laws.
Q21: A graph of the concentration of any
Q22: _ reaction is one in which two
Q23: Explain the zeroth-order reaction that takes place
Q24: Which of the following is an
Q26: Which of the following is an example
Q27: In second-order reactions, doubling the concentration of
Q28: For a given number of atoms, isotopes
Q29: The rate of decay is the decrease
Q30: A _ order reaction is one whose
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents