The following graph represents the straight-line plot to determine the rate constant of a _____-order reaction. 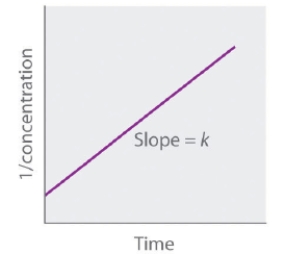
A) first
B) fourth
C) second
D) zeroth
E) third
Correct Answer:
Verified
Q31: If a plot of reactant concentration versus
Q32: Which of the following is true of
Q33: The following graph representing the concentration of
Q34: The hydrolysis of the anticancer drug cisplatin
Q35: For two or more reactions of the
Q37: The sequence of reactions that occur at
Q38: A linear change in concentration with time
Q39: The half-life of a second-order reaction under
Q40: The rate of radioactive decay is dependent
Q41: A species in a reaction mechanism that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents