In the equation given below, cyclopentanol (molar mass = 86.1 g/mol) reacts with pentanoic acid (molar mass = 102 g/mol) to produce cyclopentanoate(molar mass = 170.2 g/mol) and water. The volume of 0.297 M pentanoic acid needed for the complete reaction of 40.0 g cyclopentanol is _____ mL. 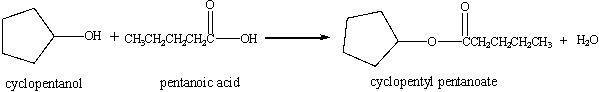
A) 0.86 × 103
B) 5.78 × 103
C) 7.58 × 103
D) 1.56 × 103
E) 0.76 × 103
Correct Answer:
Verified
Q31: The ions that do not participate in
Q32: Given: Q33: Calcium hydroxide is insoluble in water. Q34: _ is a chemical equation that shows Q35: Given: HCOOH+ KOH Q37: The net ionic equation shows only those Q38: Given: CH3CH2COOH + H2O Q39: In the reaction of barium chloride with Q40: Given: HCOOH + KOH Q41: The precipitate formed when an aqueous solution![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents